New understanding of neurons in the hippocampus: they’re all the same
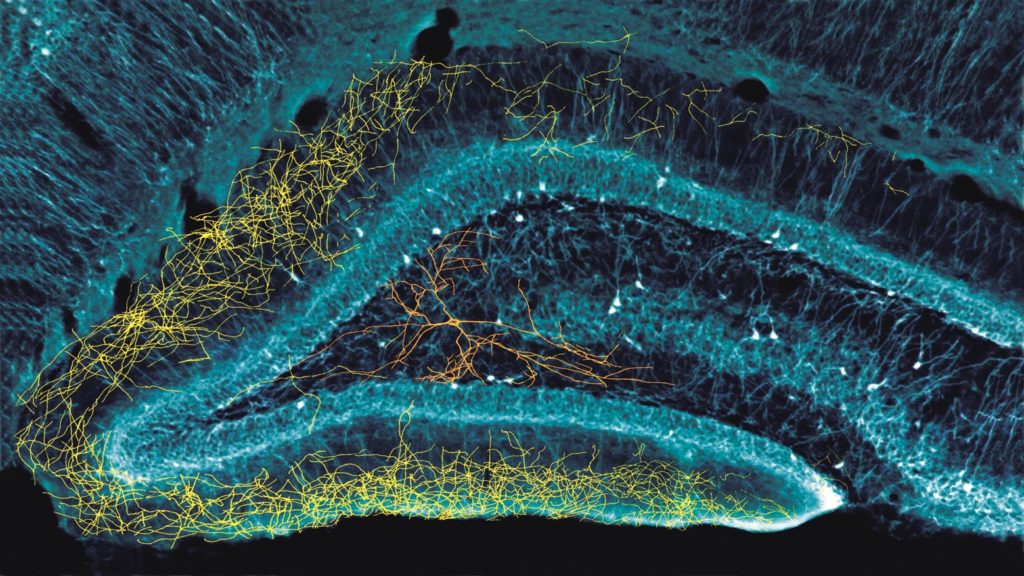
Neuroscientists at New York University School of Medicine have gained a new understanding of how neurons work in the hippocampus of the brain with the help of neural probes developed at the University of Michigan. The finding may settle a 30-year debate about the nature of seemingly inactive neurons, and whether the neurons have unique traits, as currently assumed, or whether they simply take on different functions at different times – as suggested by the research.
The experimental research is published in Science.
We had the opportunity to discuss impact of the research as well as the collaboration between engineer and neuroscientist with neuroscience team leader György Buzsáki, Biggs Professor of Neuroscience at New York University School of Medicine, and team leader of the neural probes Euisik Yoon, Professor of Electrical and Computer Engineering, and Biomedical Engineering at the University of Michigan.
This Q&A will hopefully provide a small insight into the workings of the brain, as well as an insider’s view of how the work of an engineer can help advance a neuroscientist’s ability to unravel its mysteries.
Can you give us a quick overview of your brain research?
Buzsáki: In the broadest terms, we are interested in how information is stored and transferred in the brain, in other words, how memory works.
Our central structure is the hippocampus, which is the critical part of our episodic memories. Episodic memory is our own experiences that we collect throughout our lifetime. The neurons in the hippocampus help organize the brain’s knowledge.
I, and others, believe that the hippocampus has something to do with our past and our future, but other people believe it has something to do with coding space or time, or both. In order to find the truth, we need to record from the substrate that gives rise to all of this. And in order to do that, we have to understand the conversations of neurons and the neural groups and structures and so on.
We are able to do this with Euisik’s revolutionary devices. His methods and techniques enabled a device that has neuro-sized light sources – little flashlights, so we could probe this.
And that brings us to our story in Science.
Before we get to that, can we hear a bit about the probes?
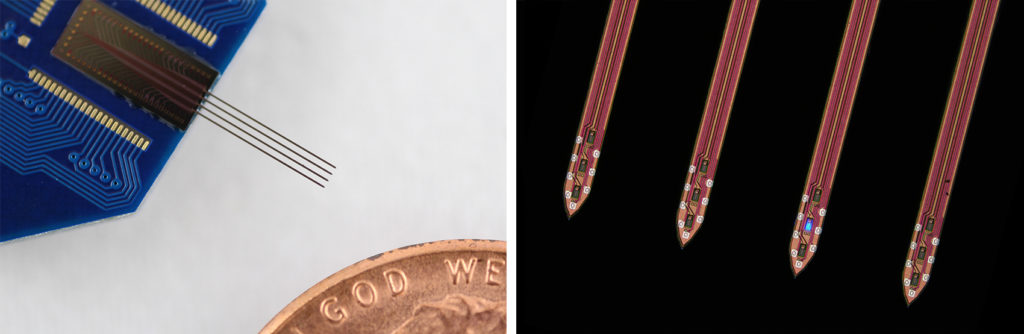
Yoon: The neural probes used in this study are equipped with LEDs, which are as small as the neurons themselves. The LEDs are capable of activating a single neuron, and the electrodes in the probe measure the electrical response of neighboring neurons. We can also activate an individual LED at precise time intervals, which is important for György’s research.
What breakthrough is described in the Science article?
Buzsáki: We found a way to get a good statistically representative sampling of brain activity by measuring subthreshold activity of neurons. This gave us a new understanding of the neurons in the hippocampus, as I’ll describe now.
In general, when we are recording, for example, 1,000 neurons – we find that 100 of them will provide information by generating action potentials [which occurs when a neuron sends some kind of information, creating an electrical charge]. But the other 90% of the neurons have very few action potentials, even after 1-2 hours of recording time.
We wanted to know what was going on with those neurons, and how they would respond to the same input. It’s something we’ve been debating for almost 30 years.
To try to find the answer, we activated those 900 neurons every few seconds using Euisik’s probes, and measured what was happening both when activated by the LEDs, and during the rest times. And we found that when we, in effect, asked the 900 neurons what they would do, they would all do the same thing as the other 10%.
This suggests that the hippocampus is not made up of different types of cells capable of doing specific jobs. For example, my group has focused on what we call memory cells [“cells” in this context refer to groups of neurons in the hippocampus that respond to specific types of inputs]. Others have focused on what they call place cells, or time cells, or border cells. Scientists have also named many other types of cells.
Euisik’s probes confirmed that they are all the same type of cell. Their different actions are driven by inputs from the neocortex.
This is a giant step in brain research – but also just a tiny step compared to what still needs to be known.
What impact does this research have in the world of brain research?
Buzsáki: Our latest research will impact at least two independent worlds in brain research. The first is the insight about whether there are many different types of neurons in the hippocampus; the experiments we conducted suggest that there is only one type.
Second, we demonstrated a way to record subthreshold activity anywhere in the brain, not just the hippocampus. Up until now, if we wanted to know something about a neuron, we would typically record the output. However, in order to interpret any information system, you have to know the input. But it’s difficult to know what the inputs are for individual neurons. Instead, they tend to group together.
For example, if I asked which political party you belong to – you might answer simply with the party name. But that disregards the decades of your life that fed into your decision – all the inputs that influenced your opinion.
It’s the same with a neuron. We want to record those inputs that are influencing the brain’s neurons.
With Euisik’s tool, we can employ what is called short time scale optogenetics. Using this technique, we gave very short light pulses separated by longer pauses. The spike responses of the neuron during the light pulses depends on the subthreshold status of that neuron, thus we could ‘read out’ indirectly the inputs to the neurons as well.
Researchers who are interested in smell, vision, motivation, emotions – whatever, can use the same method because Euisik’s electrode allows them to do large-scale measurement of subthreshold activity of neurons, without damaging the neurons.
How has the engineer-neuroscientist collaboration impacted your research?

Yoon: György has been essential in giving us an insight into what tool was needed to discover what is happening inside the brain. When we would talk, I would ask György whether what we were doing would be useful for him. And György would tell me about the problem he was trying to solve, and ask whether I could make a tool to help solve it.
Sometimes something can be fascinating as an engineering work, but useless in scientific studies. We found the happy point where engineering innovation leads to a fantastic scientific discovery. He is able to utilize the probes to a level I couldn’t even imagine.
Buzsáki: My collaboration with Michigan started with Ken Wise [William Gould Dow Distinguished University Professor Emeritus] in the late 1980s. Ken was very interested in the brain, and it’s the same for Euisik now [Euisik’s PhD advisor was Ken Wise].
We owe a lot to Euisik. He delivered a device that we can use right away, so we don’t have to be engineers. Euisik sends his students to my lab and they actually see experiments, and understand how they can answer important research questions with their own tools. Even after many years we have kept in touch with some of these students, and they are carrying on the work. This shows the social aspect of collaborations, which is so difficult to establish.
What’s Next?
Yoon: The probe that György used for this study is an earlier version of our probes. We are making next generation high density optogenetic probes that will enable neuroscientists to target neurons with a much higher resolution. I hope to deliver this probe to György and other scientists soon, and look forward to the next fantastic discovery.
Additional Information
The details of the research can be explored in the Science publication, Probing subthreshold dynamics of hippocampal neurons by pulsed optogenetics, by Manuel Valero, Ipshita Zutshi, Euisik Yoon, and György Buzsáki.
NeuroLight Technologies, a for-profit manufacturer of neurotechnology co-founded by Yoon and Wise, is a competing interest. Yoon co-authored two patents owned by the University of Michigan that cover the neural probes with optical stimulation capability.
The probes were fabricated in the Lurie Nanofabrication Facility.
Additional materials are available in the Buzsáki Lab Databank.

 MENU
MENU 
